To count chromosomes, a sample of cells is prepared and stained to visualize the chromosomes under a microscope. The chromosomes are then counted and any abnormalities in their number or structure are noted.
Advanced techniques like fluorescence in situ hybridization (FISH), karyotyping, comparative genomic hybridization (CGH), and next-generation sequencing (NGS) can provide additional information about specific regions of chromosomes or structural abnormalities.
In this article, we look at some of the most widely used techniques that not only help count chromosomes, but also detect irregularities, and their applications in research and healthcare.
Importance of Chromosomes in Genetic Study
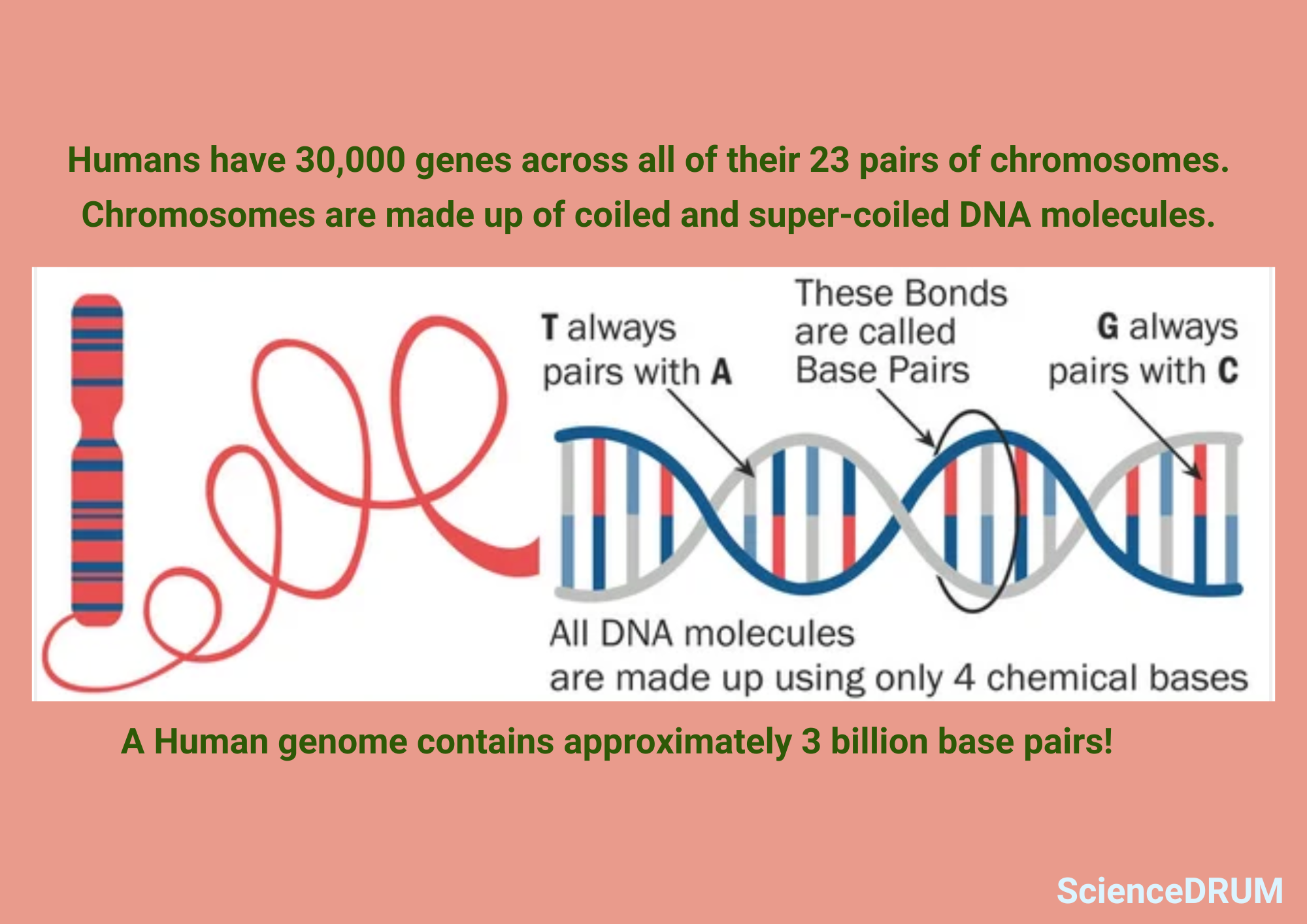
Chromosomes are the primary reason that genetic information gets transferred from one generation to another. They are long, coiled-up strands of DNA that contain all the genetic material an organism needs to develop and function.
Chromosomes determine the traits and characteristics of an organism, from physical features to susceptibility to diseases through the process of meiosis and fertilization. Without chromosomes, the information that determines an organism’s traits and characteristics would not be passed on to its offspring.
Chromosomes are also important because they help to ensure genetic stability within a cell.
During cell division, chromosomes must be accurately replicated and segregated into the daughter cells to prevent errors that could lead to genetic disorders or cancer. Any abnormalities or damage to chromosomes can have serious consequences for the cell and the organism as a whole.
In addition to their role in inheritance and genome stability, chromosomes are also important for scientific research. Studying chromosomes and their behavior during cell division can also provide insight into the mechanisms of DNA replication, gene expression, and genome stability.
Traditional techniques to identify and count chromosomes are limiting as chromosomes were visible under a light microscope only during specific stages of cell division. During other stages of cell division, they can’t be easily detected as they are not in their condensed state.
Modern techniques for analyzing and manipulating chromosomes have revolutionized many fields, including genetics, biotechnology, and medicine.
Chromosome analysis is used in prenatal diagnosis, cancer diagnosis and treatment, and identification of genetic disorders.
The field of genomics, which studies the entire set of an organism’s genes, relies heavily on chromosome analysis to map and sequence genes, and to identify variations in the genome that may make individuals susceptible to certain diseases and drug response.
How to Count Chromosomes?
Scientists use traditional as well as advanced techniques to count chromosomes.
Although traditional counting techniques are still widely used, advancements in technology have helped researchers to not only count chromosomes, but also compare multiple samples to spot structural and numerical abnormalities.
These techniques also enable scientists to focus on specific regions of the chromosomes and prevent the development of diseases like cancer or genetic disorders.
Traditional Techniques to Count Chromosomes
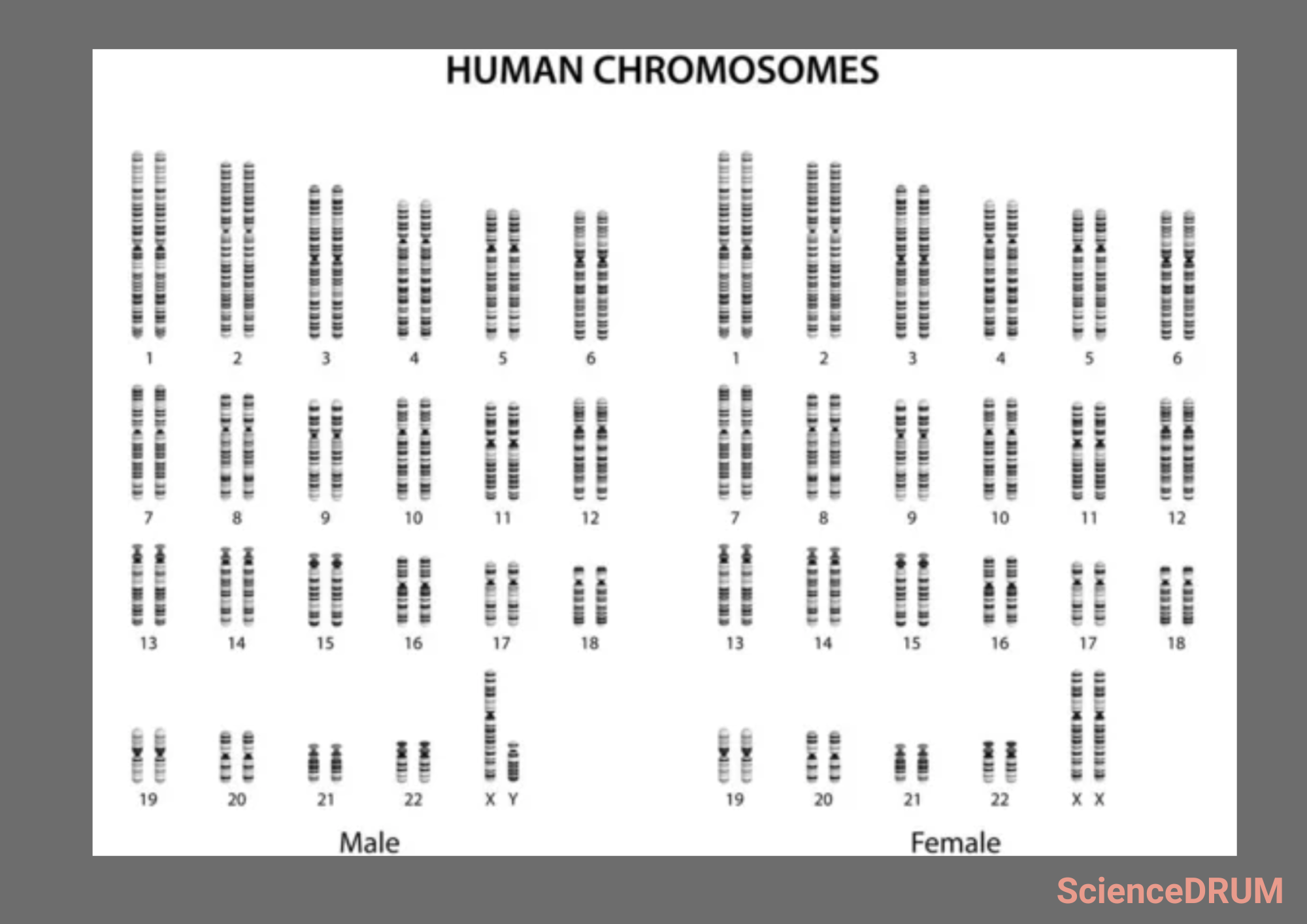
Traditional chromosome counting methods involve preparing a sample, staining the chromosomes, and observing them under a microscope to determine the number and structure of the chromosomes.
While this method is useful for locating numerical abnormalities, it has limitations when it comes to specific abnormalities or structural variations.
Giemsa staining is the most commonly used technique and produces unique banding patterns on the chromosomes that can be used to detect them.
The staining process involves treating the sample with a solution containing Giemsa dye, which binds to the chromosomes and produces a visible pattern.
Other staining techniques used in chromosome counting include Wright staining and Quinacrine staining. Once the chromosomes are visible under the microscope, the next step is to choose the right magnification and focus.
A higher magnification allows for a more detailed view of the chromosomes, but it also increases the risk of overlapping chromosomes.
It is important to adjust the focus to ensure that the chromosomes are in sharp focus, and any debris or artifacts are removed. Chromosomes can be identified by their size, shape, and banding patterns.
Each chromosome has a unique size and shape, and the banding patterns produced by staining techniques can help distinguish between them.
Chromosomes are often classified based on their banding pattern, with each band representing a specific region of the chromosome. Counting chromosomes can be a challenging task due to overlapping chromosomes or staining issues.
Some tips can make the process easier, such as keeping track of the chromosomes counted, ensuring that the chromosomes are not overlapping, and checking for abnormalities. The chromosomes are typically counted in pairs, and any abnormalities or irregularities are noted.
Modern Techniques to Count Chromosomes
Fluorescence In Situ Hybridization (FISH)
Fluorescence in situ hybridization (FISH) is a molecular biology technique that uses fluorescent probes to bind to specific DNA sequences.
By labeling specific regions of chromosomes with fluorescent probes, FISH can identify abnormalities like deletions, duplications, and translocations.
This technique can also be used to study the localization and organization of chromosomes within the nucleus. FISH is commonly used in cancer research to find specific genetic abnormalities that may be driving the development of the tumor.
Karyotyping
Karyotyping is a technique that involves arranging the chromosomes in a specific order and studying their structure and organization.
Karyotyping can reveal structural abnormalities like inversions, translocations, and ring chromosomes that may not be visible with traditional chromosome counting methods.
Karyotyping can also uncover numerical abnormalities like aneuploidy, where cells have an abnormal number of chromosomes. Karyotyping is commonly used in clinical settings to diagnose genetic disorders like Down syndrome or Turner syndrome.
Comparative Genomic Hybridization (CGH)
CGH is a technique that uses DNA microarrays to compare the DNA content of two different samples.
By comparing the DNA content of a normal sample to a sample with a suspected abnormality, CGH can identify regions of the genome that may be deleted, duplicated, or amplified in the abnormal sample.
This technique is commonly used in cancer research to reveal genetic abnormalities that may be driving the development of the tumor.
Array-based comparative genomic hybridization (aCGH) is a newer version of CGH that uses microarrays with a higher density of probes.
This technique can pinpoint smaller regions of the genome that may be involved in genetic abnormalities. aCGH is commonly used in clinical settings to diagnose genetic disorders like intellectual disability or developmental delays.
Next-generation Sequencing (NGS)
NGS is a high-throughput sequencing technique that can sequence millions of DNA fragments simultaneously. NGS can be used to study the genome in detail, including detecting genetic variants and structural abnormalities.
NGS is commonly used in research to study the genetic basis of complex diseases like cancer or neurological disorders. Single-cell sequencing is a newer version of NGS that allows researchers to sequence the DNA from individual cells.
By sequencing the DNA from individual cells, researchers can study genetic diversity and heterogeneity within a population of cells. Single-cell sequencing is commonly used in cancer research to identify genetic subpopulations within a tumor that may be resistant to therapy. {1} {2}
How to Interpret and Report Chromosome Count Results?
Interpreting and reporting chromosome count results requires knowledge of the normal number and structure of chromosomes in the species being studied. In humans, for example, a normal diploid cell should have 46 chromosomes.
Any deviations from this number may indicate a chromosomal abnormality. Structural abnormalities like translocations, inversions, or deletions can also be identified through careful analysis of the chromosome structure.
Once the chromosome count and any abnormalities have been identified, the results can be reported in a clear and concise manner.
The report should include the number of chromosomes counted, any numerical or structural abnormalities identified, and any additional information gathered through advanced techniques like FISH or karyotyping.
Reports should include interpretation and recommendations for further testing or follow-up. For example, if a numerical abnormality is identified, further testing may be necessary to determine whether the abnormality is present in all cells of the body or only in a subset of cells.
This can have implications for the severity of the condition and the likelihood of passing the abnormality on to offspring.
Clear and accurate reporting of chromosome count results improves communication between healthcare professionals, researchers, and patients and leads to better treatment decisions during genetic counseling.
It’s also important to consider ethical and legal implications of sharing and storing chromosome count results, as well as protecting the privacy of the patient and their genetic information.
Frequently Asked Questions
Sources:
1 – Bioessays: “How to count chromosomes in a cell: An overview of current and novel technologies.”
2 – Genome Biology and Evolution: “The Evolution of Chromosome Numbers: Mechanistic Models and Experimental Approaches.”